-
Courses

Courses
Choosing a course is one of the most important decisions you'll ever make! View our courses and see what our students and lecturers have to say about the courses you are interested in at the links below.
-
University Life

University Life
Each year more than 4,000 choose University of Galway as their University of choice. Find out what life at University of Galway is all about here.
-
About University of Galway

About University of Galway
Since 1845, University of Galway has been sharing the highest quality teaching and research with Ireland and the world. Find out what makes our University so special – from our distinguished history to the latest news and campus developments.
-
Colleges & Schools

Colleges & Schools
University of Galway has earned international recognition as a research-led university with a commitment to top quality teaching across a range of key areas of expertise.
-
Research & Innovation

Research & Innovation
University of Galway’s vibrant research community take on some of the most pressing challenges of our times.
-
Business & Industry

Guiding Breakthrough Research at University of Galway
We explore and facilitate commercial opportunities for the research community at University of Galway, as well as facilitating industry partnership.
-
Alumni & Friends

Alumni & Friends
There are 128,000 University of Galway alumni worldwide. Stay connected to your alumni community! Join our social networks and update your details online.
-
Community Engagement

Community Engagement
At University of Galway, we believe that the best learning takes place when you apply what you learn in a real world context. That's why many of our courses include work placements or community projects.
Proteins at Liquid Interfaces
Protein adsorption, structure and function at liquid interfaces
Being made of both hydrophilic and hydrophobic amino acids proteins are intrinsically amphiphilic molecules and will readily adsorb onto interfaces between water and non-polar fluids (such as oil-water and air-water interfaces). In most biological systems this is typically avoided as changes in protein structure that occurs is typically associated with a loss in function. Adsorption onto such interfaces, however, is often unavoidable in industrial and biotechnological processes so understanding the link between protein function and structure at interfaces is important in the applications of proteins and other biomolecules.
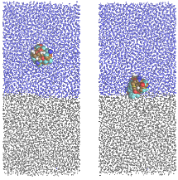
Snapshots taken from simulation of HFBII hydrophobin at water-octane interface (taken from DL Cheung, Langmuir, 28, 8730, 2012)
In order to investigate this link the group is currently using molecular dynamics simulation to investigate proteins at fluid interfaces. Initilly this focused on biosurfactant proteins (proteins that act as detergents) such as the hydrophobins. Using simulation we have investigated that link between the structure of these proteins and their interfacial behaviour, in particular examining the effect of their amphiphilic surface structure on the strength of their adsorption at an oil-water interface.
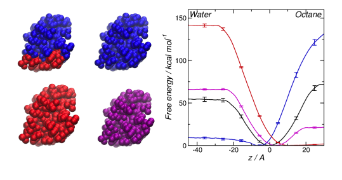
Effect of surface structure on interface between HFBII and water-octane interface (taken from DL Cheung, Langmuir, 28,8730, 2012)
More recently we have started investigating protein structure at liquid interfaces. When a protein adsorbs onto a liquid interface it will commonly undergo conformational change, as the hydrophobic amino acids that normally reside in the core of protein partition into the hydrophobic (air/oil/etc) medium. The try to understand the link between protein structure and interfacial conformation and function we performed simulations of some peptides derived from the protein myoglobin. For the two peptides we investigated we found that these exhibited a number of different conformations at the air-water interface; these different conformations were similar in energy to each other but separated by relatively large energy barriers. One of these, consisting of the first 55 residues of the full protein, typically formed extended conformations. The other peptide was more commonly found in compact states. This difference in the preferred conformation is consistent with the differing emulsification properties of these proteins, as the first protein was found to be a more effective emulsifier than the second.
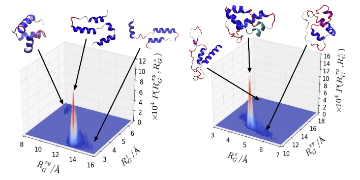
Conformations of myoglobin-derived peptides at air-water interface (taken from DL Cheung, Langmuir, 32, 4405, 2016)















