-
Courses

Courses
Choosing a course is one of the most important decisions you'll ever make! View our courses and see what our students and lecturers have to say about the courses you are interested in at the links below.
-
University Life

University Life
Each year more than 4,000 choose University of Galway as their University of choice. Find out what life at University of Galway is all about here.
-
About University of Galway

About University of Galway
Since 1845, University of Galway has been sharing the highest quality teaching and research with Ireland and the world. Find out what makes our University so special – from our distinguished history to the latest news and campus developments.
-
Colleges & Schools

Colleges & Schools
University of Galway has earned international recognition as a research-led university with a commitment to top quality teaching across a range of key areas of expertise.
-
Research & Innovation

Research & Innovation
University of Galway’s vibrant research community take on some of the most pressing challenges of our times.
-
Business & Industry

Guiding Breakthrough Research at University of Galway
We explore and facilitate commercial opportunities for the research community at University of Galway, as well as facilitating industry partnership.
-
Alumni & Friends

Alumni & Friends
There are 128,000 University of Galway alumni worldwide. Stay connected to your alumni community! Join our social networks and update your details online.
-
Community Engagement

Community Engagement
At University of Galway, we believe that the best learning takes place when you apply what you learn in a real world context. That's why many of our courses include work placements or community projects.
NBL News
Latest News:
This page contains, recent and old news, and a section of graduation photos.
12/11/2025: Latest paper published online.This research was part of Dr. Bernard Boateng's PhD project.
Abstract: Background: Accurate monitoring and control of bioprocesses require the measurement of critical parameters, such as product and interfering protein concentrations, particularly in the bioreactor harvest prior to during downstream purification. One promising approach is to combine polarised multidimensional fluorescence measurements of intrinsic emission with light scattering measurements. This offers a non-invasive and sensitive method for protein quantification that can be implemented using either polarised Excitation Emission Matrix (pEEM) or Total Synchronous Fluorescence (pTSFS) measurement modes which can deal with matrix interferences from the complex cell culture media which is also fluorescent. Results: Polarised Synchronous Light Scattering (pSyLS), pTSFS, and pEEM measurements on a sample set of 45 unique samples with varying protein (Immunoglobulin G and Bovine Serum Albumin) and media (yeastolate) concentrations (the model for a clarified, multi-protein bioreactor harvest) were made. Spectral data were evaluated using Principal Component Analysis (PCA) and Partial Least Squares (PLS) regression used for quantifying IgG and total protein content. Best results were achieved with parallel polarised (||) measurements for both total protein content, prediction errors of 11%, 14%, and 14% using TSFS||, EEM||, and SyLS|| measurements respectively, and IgG content, with prediction errors of 10% for both TSFS|| and EEM||. Quantification was best when the Rayleigh-Mie scatter signal was included. The primary limitations were a combination of low sample numbers (n=45) along with a relatively low target analyte range (max 1.8 gL–1), with reference method accuracy being a secondary consideration. Significance & Novelty: Demonstrated for the first time that pSyLS can be used for protein quantification in a complex clarified bioreactor harvest model. This is also the first comparison between polarised TSFS, SyLS, and EEM measurements for estimating total protein and IgG content in a complex interfering matrix. For pEEM measurements the significant result is that for samples with a constrained particle size distribution, including Rayleigh-Mie scatter signal in the model enables better protein quantification. This shows the strong potential of pSyLS, pTSFS, and pEEM measurements for real time bioprocess monitoring of protein content. This research was supported by a research grant from Science Foundation Ireland (SFI) and is co-funded under the European Regional Development Fund under Grant number (14/IA/2282, Advanced Analytics for Biological Therapeutic Manufacture, to AGR) |
06/11/2025: Latest instrumentation, part 5.
This was installed on the 4/11/2025 and is being used to support our work on high concentration protein formulations. |
20/08/2025: Latest instrumentation, part 4.
|
11/08/2025: Latest instrumentation, part 3.
We're using it for both filtration and protein concentration based experiments. |
20/03/2025: Latest instrumentation, part 2.
|
20/03/2025: Latest instrumentation.
This is an Anton-Paar Litesizer 500 and it's been acquired to complement the other DLS system and facilitate the setting up of two Aqualog/DLS workstations. |
27/01/2025: Latest paper published.Nano- and Meso-scale aggregation of Poly(N-isopropylacrylamide) below the Lower Critical Solution Temperature: a wide-angle Dynamic Light Scattering study. M. de Castro and A.G. Ryder.
All samples were polydisperse and accurate particle size distribution (PSD) data was only obtained from distribution fitting, being consistent and accurate down to ∼ 0.1 wt%. In water at 1 wt%, Rh, extracted from distribution fitting: 12.4 ± 0.6 nm (55 kDa), 10.0 ± 0.22 nm (38 kDa), 6.2 ± 0.15 nm (28.5 kDa), and 9.7 ± 0.14 nm (20–25 kDa) were significantly higher than that expected for single PNIPAm chains in solution. Measurements in different buffers of varying pH (7.4–5.0) yielded similar sizes (Rh of 6–15 nm) and polydispersity indicating that these were stable aggregates. These aggregates could be broken down with Triton-X but not with sodium dodecyl sulphate, ultrasound, or by heating above the LCST and then cooling. We suggest that this nanoscale aggregation and increased polydispersity was caused a variety of factors including by solid-state aging during prolonged storage (>5 years) induced by water adsorption, and/or manufacturing processes.
Stirring was found to produce larger, meso-scale (Rh > 150 nm), soluble aggregates and the rate of formation of these meso-particles was linear with stirring time (with a concomitant linear decrease in the faction of original nanoscale aggregates). Meso-particle formation was not correlated with MW, but was inversely correlated to polymer concentration suggesting that aggregation was driven by adsorption at air/liquid interfaces rather than solution phase collisions. In conclusion, PNIPAm particle size and distribution was highly dependent on multiple factors including source, storage conditions, and exposure to air–water interfaces. Standard wide angle DLS is however an effective and rapid method for identifying and quantifying PNIPAm aggregation.
|
19/11/2024, Postdoc vacancy.Vacancies updated: 18/11/2024, Postdoc position available.Applications are invited from suitably qualified candidates for a full time Postdoctoral Researcher position with the Nanoscale Biophotonics Laboratory at the University of Galway. |
10/09/2024, Latest toy in the lab.
|
25/06/2024, Latest paper published.Our latest biomaterials analysis paper has just been published. This was part of Camila Van Zanten's PhD thesis research. Estimating Poly(N-isopropylacrylamide) size in solution below the LCST using Fluorescence Correlation Spectroscopy with non-covalent bound fluorophores.C. van Zanten and A.G. Ryder. Polymer Testing, 137, 108500, (2024). DOI: 10.1016/j.polymertesting.2024.108500 Abstract: Accurate size measurements of nanosized polymer chains in dilute solutions is important for understanding polymer behavior, however, these measurements can be challenging to implement accurately, and are technique dependent. Here we explore the use of Fluorescence Correlation Spectroscopy (FCS) to determine the size of single polymer chains in dilute solutions (<2 wt%). FCS, a technique with single molecule sensitivity, generally requires the use of covalently labelled polymers which can distort physicochemical behavior. Here, FCS based size measurements were based on the non-covalent interaction of fluorophores (Alexa 405, Atto 390, and Atto 425) with PNIPAm in water at 25 °C (below the Lower Critical Solution Temperature). FCS estimated size (hydrodynamic radius) of three different MW PNIPAm samples in water were: 5.0 ± 1.0 nm (28.5 kDa), 5.0 ± 1.0 nm (38 kDa), 3.3 ± 0.5 nm (55.5 kDa), in reasonable agreement with theoretical calculations. Accuracy was directly related to the fraction of PNIPAm-bound fluorophore, which were, for 1 wt% PNIPAm solutions in water: ∼11.5 % (Atto 390), ∼8.1 % (Atto 425), and 4 % (Alexa 405). This method has several advantages in that it does not require a covalent labelling of PNIPAm, it can be implemented on very small sample volumes, and allows for in-situ measurements. |
27/05/2024: Downstream Protein Analysis - Polarized Emission Spectroscopy (DPA-PES).I'm glad to announce our latest project in the bioanalytical science domain. The DPA-PES project aims to develop a range of novel measurement methodologies based on polarised Excitation Emission Matrix (pEEM) spectroscopy for the quantitative analysis of therapeutically relevant biological molecules such as monoclonal antibodies (mAbs). The project is funded by Science Foundation Ireland , under grant 22/FFP-A/10271, and will run for 5 years from January 2024. |
22/03/2024: Online PhD viva presentation by Shane Grant.Title: Elemental Screening of Cell Culture Media using Microwave Plasma Atomic Emission Spectroscopy.Time: 2:00 pm am (Irish Time), 22/03/2024. Shane will be presenting his PhD research online via an Microsoft Teams Live Event. Abstract: Biopharmaceutical manufacturing often faces process variability due to varying raw materials between production batches. This variability can affect the quality and yield of biologics, despite efforts to use chemically defined media (CDM). CDM contains many components (n>30) and small concentration differences in some components (particularly metals) can unpredictably affect production outcomes, such as low yields or product rejection. To identify lot-to-lot CDM variability, a screening method suitable for complex media is required. Inorganic salts, the largest component in media, serve critical roles in many cellular functions. Conventional techniques such as ICP-OES and ICP-MS, are however, costly, require experienced operators, and are not generally implemented. |
14/02/2024, PhD studentships available.Applications are invited for two, funded PhD research positions commencing between April and September 2024. The posts are part of a larger comprehensive project that involves investigating and developing the use of novel 4 and 5-dimensional fluorescence measurement methods for the quantitative analysis of macromolecules in complex environments, particularly for Downstream Processing (DSP). The research straddles both fundamental and applied research domains and builds on our recent investigations which showed that polarized Excitation Emission Matrix (pEEM) measurements which is combination of scatter and polarized emission, provides a unique methodology for monitoring simultaneously both physical and chemical changes in therapeutic proteins. More details can be found on: www.universityofgalway.ie/nanoscale/vacancies/ |
07/09/2023, Latest paper accepted.Our latest media analysis paper has just been accepted, this was part of some research that was done in conjunction with GSK (USA). The pre-print is now available online: Abstract: Yeastolate is often used as a media supplement in industrial mammalian cell culture or as a major media component for microbial fermentations. Yeastolate variability can significantly affect process performance, but analysis is technically challenging because of compositional complexity. However, what may be adequate for manufacturing purposes is a fast, inexpensive screening method to identify molecular variance and provide sufficient information for quality control purposes, without characterizing all the molecular components. Here we used Size Exclusion Chromatography (SEC) and chemometrics as a relatively fast screening method for identifying lot-to-lot variance (with Principal Component Analysis, PCA) and investigated if Partial Least Squares, PLS, predictive models which correlated SEC data with process titer could be obtained. SEC provided a relatively fast measure of gross molecular size hydrolysate variability with minimal sample preparation and relatively simple data analysis. The sample set comprised of 18 samples from 12 unique source lots of an ultra-filtered yeastolate (10 kDa molecular weight cut-off) used in a mammalian cell culture process. SEC showed significant lot-to-lot variation, at 214 and 280 nm detection, with the most significant variation, that correlated with process performance, occurring at a retention time of ~6 minutes. PCA and PLS regression correlation models provided fast identification of yeastolate variance and its process impact. The primary drawback is the limited column lifetime (<300 injections) caused by the complex nature of yeastolate and the presence of zinc. This limited long term reproducibility because these age-related, non-linear changes in chromatogram peak positions and shapes were very significant.
|
07/09/2022, Latest paper accepted & published online.
Abstract: Antibody-drug Conjugates (ADCs) are promising anticancer therapeutics, which offer important advantages compared to more classical therapies. There are a variety of ADC Critical Quality Attributes (CQAs) such as the protein structure, aggregation, and drug-to-antibody ratio (DAR), which all impact on potency, stability, and toxicity. Production processes can destabilize antibodies via a variety of physical and chemical stresses, and via increased aggregation after conjugation of hydrophobic drugs. Thus, a proper control strategy for handling, production, and storage is necessary to maintain CQA levels, which requires the use of in-process quality measurements to first identify, then understand, and control the variables which adversely affect ADC CQAs during manufacturing. Here we show how polarized Excitation Emission Matrix (pEEM), a sensitive, non-destructive, and potentially fast technique, could be used for rapidly assessing aggregation and DAR in a single measurement. pEEM provides several sources of information for protein analysis: Rayleigh scatter for identifying aggregate/particle formation and fluorescence emission to assess chemical and structural changes induced by attachment of a linker and/or a small molecule drug payload. Here we used a non-toxic ADC mimic (monoclonal antibody with linker molecule) to demonstrate efficacy of the measurement method. Emission changes caused via light absorption by the attached linker, allowed us to predict DAR with good accuracy using fluorescence signal from the final purified products (6% relative error of prediction (REP)) and also from unpurified alkylation intermediates (11% REP). pEEM changes could also be correlated with size (hydrodynamic radius, Rh) and aggregate content parameters obtained from Dynamic Light Scattering and Size Exclusion Chromatography (SEC). For the starting material and purified product samples, pEEM correlated better with Rh (R2 =0.99, 6% REP) than SEC determined aggregate content (18% REP). Combining both fluorescence and light scatter signals also enabled in-process size quantification (6% REP). Overall, combining polarized measurements with EEM and Rayleigh scatter provides a single measurement, multi-attribute test method for ADC manufacturing. |
|
22/10/2020: Online PhD viva presentation by Bernard O. Boateng. Title: Bioprocess Monitoring using Polarised Multidimensional Fluorescence Spectroscopy.Time: 9:00 am (Irish Time), 25/05/2022. Bernard will be presenting his PhD research online via an Microsoft Teams Live Event. This is a public event and can be accessed by clicking on this link. Abstract: Bioprocess monitoring is essential for biopharmaceutical manufacturing as it helps to ensure the required quality of the therapeutic proteins and maximise the productivity of the bioprocess as per Quality-by-Design (QbD) principles. The monitoring of critical process parameters (CPPs) such as viable cell density and protein quantification is complicated by the complex chemical composition of the bioreactor broth. This thesis presentation covers the systematic development and application of polarised multidimensional fluorescence (pMDF) spectroscopy as an analytical technique for bioprocess monitoring. Published work: Development of a rapid Polarized Total Synchronous Fluorescence Spectroscopy (pTSFS) method for protein quantification in a model bioreactor broth. B.O. Boateng, S. Elcoroaristizabal, and A.G. Ryder, Biotechnology and Bioengineering, 118(5), 1805-1817, (2021).[Open access] DOI: 10.1002/bit.27694 |
Dark days for Ukraine and Europe, shades of 1939.
Appeasing corrupt regimes always leads to death & destruction. Putin is a dictator in all but name and has ill served the Russian people over the past 20 years. His goal will be to first subjugate Ukraine and it will not stop there. Those who do not learn from history are condemned to repeat the mistakes of the past...appeasement did not, and does not work. |
29/12/2021: Latest paper.
|
06/07/2021: Another prize winning NBL PhD student!! Two in a week.
This research was supported by a research grant from Science Foundation Ireland (SFI) and is co-funded under the European Regional Development Fund under Grant number (14/IA/2282, Advanced Analytics for Biological Therapeutic Manufacture, to AGR) |
01/07/2021: Another prize winning NBL PhD student.
|
15/06/2021: Newly minted PhDs.
Marina and Ana gowned up for the occasion. |
14/05/2021: Online Technology webinar on 21/05/2021.
I am happy to announce we are holding the first of the PAT4Nano technology webinars next Friday online (1-3 pm UTC, 21/05/2021) as a Teams Live Event. |
22/04/2021: PAT4Nano website
I am happy to announce that the PAT4Nano website (https://pat4nano.com) has just gone live. Here you can read all about the tools we are developing for online/real-time nanoparticle characterization in suspensions. |
28/01/2021: Latest paper.I'm pleased to announce that our latest paper has just been accepted in Biotechnology & Bioengineering. Congrats to Bernard on his first thesis paper. Development of a rapid Polarized Total Synchronous Fluorescence Spectroscopy (pTSFS) method for protein quantification in a model bioreactor broth. B.O. Boateng, S. Elcoroaristizabal, and A.G Ryder, Biotechnology and Bioengineering, Online, accepted version, 27/01/(2021). DOI: 10.1002/bit.27694 |
|
|
24/11/2020: PAT4Nano webinar: Realtime Nanoparticle Measurements, an introduction to PAT4Nano: solving industrial needs using innovative solutions.
The PAT4Nano consortium is pleased to host this introductory webinar about online nanoparticle measurements and multi-PAT solutions for nanomaterial production. The aim of this web conference is to bring together scientists, researchers, engineers, and industry to share their experience, gain and evaluate emerging technologies in nanosuspension and nanoparticle analysis. Time: Tuesday 24th of November 2020, from 3PM (UTC). |
24/10/2020: Latest paper.
|
22/10/2020: Online PhD viva presentation by Ana Luiza de Faria e Silva.
Time: 9:00 am (Irish Time), 22/10/2020. |
08/09/2020: Latest paper.Our latest pEEM paper has just been accepted by Analytica Chimica Acta, and is now available here: DOI: 10.1016/j.aca.2020.09.007. Quantitative analysis of weakly-bound insulin oligomers in solution using polarized multidimensional fluorescence spectroscopy. Y. Casamayou-Boucau and A.G. Ryder. Analytica Chimica Acta, 1138, 18-29, (2020). DOI: 10.1016/j.aca.2020.09.007. This is an open access paper. |
13/07/2020: Latest paper.Our latest paper has just been accepted by Biotechnology and Bioengineering, and the accepted article is available on the website: DOI: 10.1002/bit.27483 Characterization of lysozyme PEGylation products using polarized excitation‐emission matrix spectroscopy. A.-L. de Faria e Silva, S. Elcoroaristizabal, and A.G. Ryder. Biotechnology and Bioengineering, in press, (2020). DOI: 10.1002/bit.27483 |
05/06/2020 BOC award to Marina Steiner-Browne.
This research was supported by a research grant from Science Foundation Ireland (SFI) and is co-funded under the European Regional Development Fund under Grant number (14/IA/2282, Advanced Analytics for Biological Therapeutic Manufacture, to AGR)
|
27-28 May 2020: PAT4Nano Virtual Kick Off Meeting.
|
Nov. 2019: DLS system installed.Our new Dynamic Light Scattering (DLS) system was installed this month. We acquired a Zetasizer Nano ZS (Malvern Pananalytical) to support activities related to the analysis of proteins, specialty nanomaterials and small molecule APIs. |
23/10/2019: Latest paper.Our latest paper has just been published in Chemometrics and Intelligent Laboratory Systems. Using Polarized Total Synchronous Fluorescence Spectroscopy (pTSFS) with PARAFAC analysis for Characterizing Intrinsic Protein Emission. M. Steiner-Browne, S. Elcoroaristizabal, and A.G. Ryder. Chemometrics and Intelligent Laboratory Systems, 194, 103871, (2019). DOI: 10.1016/j.chemolab.2019.103871 The paper can be downloaded free of charge from here until 12/1219. |
11/June/2019
|
16/April/2019
More details about BioPharmaChem can be found at www.pharmachemskillnet.ie/ |
06/Mar./2019
This research was supported by a research grant from Science Foundation Ireland (SFI) and is co-funded under the European Regional Development Fund under Grant number (14/IA/2282, Advanced Analytics for Biological Therapeutic Manufacture, to AGR). |
21/Feb./2019
|
28/Nov./2018Congratulations to Przemyslaw Zarski who passed his viva voce examination on Wednesday. His thesis was entitled: Using Total Internal Reflection Fluorescence Microscopy (TIRFM) for studying Protein-surface interactions. He is now working at Abbott in Longford. |
04/Sept./2018:We have recently had two papers accepted and published in Langmuir and Methods and Applications of Fluorescence:
|
11/April/2018:
|
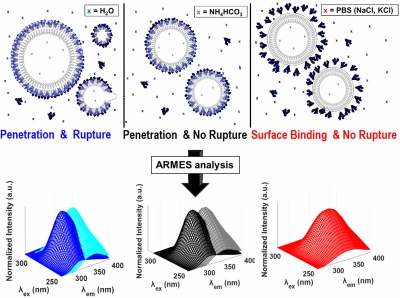
06/March/2018:
His presentation was focused on the use of Anisotropy Resolved Multi-Dimensional Emission Spectroscopy (ARMES) for measuring Insulin aggregation. Yannick's research was funded by an Irish Research Council postgraduate scholarship award (#GOIPG/2015/3826). |
Feburary 2018:
|
Graduation Photos:
| Some Pics from various PhD graduations from the group. | |
 |
 |
| Dr. Przemyslaw Zarski (left) and Dr. Yannick Casamayou-Boucau, 11th June 2019. | Dr. Bernard Boateng's graduation 2022. |
 |
 |
| Dr. Michelle Kyne, 23/03/2017 (with cousin Dr. Ciara Kyne). | Dr. Michelle Hennigan (2014). |
 |
 |
| Dr. Bridget Kissane | Dr. Amandine Calvet. |
To be added.
Older News:
- Aug. 2017: Our new elemental characterization facility, a Microwave Plasma, Atomic Emission Spectrometer (MP-AES) has been installed and is undergoing commissioning studies. Mostly going to be applied to analytical research for biopharmaceutical manufacturing.
- 07/03/2017: Our new Supercontinuum laser from Leukos was installed today. This laser source will be used for advanced fluorescence lifetime spectroscopy.
- July 2016: The Single Molecule Detection facility has now been upgraded and recommissioned. This is now set up for a variety of experimental methods including FCS, PCH, FLIM, etc.
- 02/03/2016: Congratulations to Dr. Radu Groza who successfully defended his PhD thesis at his viva exam today.
- 27/04/2015: Awarded SFI Principal Investigator award. Details here. We will be advertising at least two postdoc and two PhD positions in 2015 on this project (Details on my vacancies page). In 2016 we will be advertising a further two PhD research posts.
- 17/03/2015: Paper published in Analytical Chemistry: Low-content quantification in powders using Raman spectroscopy: a facile chemometric approach to sub 0.1% limits of detection., B. Li, A. Calvet, Y. Casamayou-Boucau, C. Morris, and A.G. Ryder, Analytical Chemistry, 87(6), 3419-3428, (2015). DOI: 10.1021/ac504776m
- 30/09/2014: I have moved office to R213 in the Physical Chemistry Section.
- 07/01/2014: Congratulations to Dr. Michelle Hennigan who successfully defended her PhD thesis at her viva exam today.
- 11/06/2013: We have just received a PhAT Imaging microscopy imaging system from Kaiser Optical Systems Inc. This system is being evaluated for use in Real-Time-Release applications of small molecule APIs and also for the analysis of complex cell culture media. This research is being carried"out" under the auspices of the EI-IDA PMTC project.
- 30/11/2012: Congratulations to Amandine Calvet who successfully defended her PhD thesis at her viva exam today.
- 21/11/2012: Congratulations to Domhnall O'Shaughnessy who graduated with his PhD today.
- Oct. 2012: We are about to embark on a new research effort as part of the Enterprise Ireland/IDA Pharmaceutical Manufacturing Centre. As part of this centre we aim to develop some novel chemometric and spectroscopic tools to enable the Real-Time-Release of APIs. As part of this project we will be collaborating closely with Kaiser Optical Systems Inc. More information will follow in due course.
- Oct. 2012: Three new PhD students have commenced their research projects here, they are Michelle Kyne (Galway), Radu Groza (Romania), and Przemyslaw Zarski (Poland). They will be studying the photophysics of triazine fluorophore interactions, novel bioanalytical methods for complex materials analysis, and protein-surface interactions respectively.
- 20/06/2012: Our Avalon RamanStation has now surpassed the 250,000 spectra count. As of this morning the count is at 264,225 spectra saved.
- 13/06/2012: Congratulations to Domhnall O'Shaughnessy who successfully defended his PhD thesis at his viva voce examination today.
- April 12: Congratulations to Michelle Hennigan (PhD student) who will shortly be starting a new job with Merck in the UK.
- 23/03/2012: Dr. Cheryl Morris was conferred with her PhD. She researched the use of fluorescence methods for characterizing thermoresponsive polymers.
- Feb. 2012: Michelle Hennigan was one of the two winners of the BOC postgraduate award.
- Jan. 2012: New water activity meter installed in the lab. We have just acquired an Aqualab Water Activity meter 4TE for bio-analytical applications.
- Sept. 2011: Amandine Calvet a PhD student in the Nanoscale Biophotonics Laboratory was awarded a best poster prize at the 12th Conference on Methods and Applications of Fluorescence: Spectroscopy, Imaging and Probes (MAF-12) held in Strasbourg, France, from 11 to 14 Sept., 2011. Amandine (pictured below with her certificate) was awarded a 200 euro book voucher for her poster entitled: Quantification of amino acids and vitamins in cell culture media using Excitation-Emission Matrix fluorescence spectroscopy and chemometrics. A. Calvet and A.G. Ryder. She was one of only 6 prize recipients from a total of 227 posters presented by PhD students, postdocs, and academic staff.
- May 2011: The MolRec UREKA programme will begin in the next couple of weeks. Two third year students, Rebecca Pigot and Michelle Kyne will be joining the lab for the summer to work on the spectroscopic analysis of complex bioprocess media.
- Feb. 2011: We have just started a new collaboration with Kaiser Optical Systems Inc. and are evaluating the application of their new RAMANRXN2 1000 analyzer system for biopharmaceutical applications. This is part of a wider collaboration with UCD, DCU, and NIBRT.
- Jan. 2011: Our work on rapid cell culture media analysis has been featured in the January issue of Genetic Engineering & Biotechnology News. The article can be accessed here.
- Oct. 2010: Paper of fluorescence analysis of polymers published in Macromolecules.
- Sept. 2010: A new industrial collaboration with the Janssen Pharmaceutical Companies of Johnson & Johnson began on the 1st of September. The project, funded by J&J and NIBRT is developing new methods for characterizing cell culture media. A press release is available to download from here.
- Summer 2010: We have a new group of summer students joining us this year they include MSc students Denis Coffey and Caroline Golderick, and UREKA students Jonathon Donaghue and Ronan Fitzpatrick.
- Mar. 2010: Congratulations to Dean St. Mart from NUIM, a postgrad in Dr. Jon Stephens group who has been selected to attend the 60th Lindau Nobel Laureates Meeting in June. We are collaborating with Dean and John on the characterization of novel fluorophores which are synthesized in NUIM.
- Summer 2009: We have a new group of summer students joining us this year they include MSc students Loretta Breslin and Neil Murphy, Cesar Maule PhD student from Oporto, Angela Chang, Nadine McCleary & Edel Houghton, and Valerie Murphy.
- 23 Oct. 2008: "TIRF's UP": The Total Internal reflection Fluorescence (TIRF) Microscope has now been installed and is up and running. It is located in R166 has has a triple laser excitation unit, MT20 white light excitation, EMCCD detector, and a x60 TIRF objective (oil immersion).
- 15 Oct. 2008: We have now relocated the laboratories into the School of Chemistry and the microscopy lab. is also now operational.
- Aug. 2008: New microscopy lab under development: The new microscopy lab for the NBL is currently being refurbished and we hope to move in late September. We intend to co-locate all 5 confocal and single-molecule microscopy systems in a single dedicated location in the School of Chemistry. This facility will comprise of a dedicated room for the confocal live cell imaging and in-vivo spectroscopic measurements, and a separate lab for the materials science and TIRF setups. This new facility will be air-condition and optimised for all our microscopy needs. Will post more details later.
- Aug. 08: Postdoctoral position in spectroscopy & chemometrics available now for immediate start.
- Aug. 08: Postgraduate studentship available in the area of pharmaceutical analysis.
- May 08: New Thor Labs Laser Scanning Confocal Microscope (LCSM) installed in lab. More details here.
- Mar. 08: New FT-IR, UV-Visible, and TCSPC instrumentation installed in laboratory.
- Feb. 08: Spectroscopy notes for 3Y 2008 now uploaded.
- Jan. 08: The website has now been totally updated. We are adding new pages and information about facilities, lectures, projects, etc.
- Dec. 07: The NBL has just been awarded funding to acquire a Total Internal reflection Fluorescence (TIRF) Microscope from Science Foundation Ireland. This will considerably enhance our ability to measure dynamic processes on the nanoscale.
- Oct 07: The NBL has been part funded under the National Biophotonics Imaging Platform (a collaborative advanced imaging consortium). As part of our contribution we will collaborate with partners in the RCSI and other institutions by providing our expertise and instrumentation in FCS, FCCS and other advanced fluorescence methods. The NUIG element is a collaboration between Prof. Peter Dockery of the Department of Anatomy, Prof. Chris Dainty, Applied Optics, Department of Experimental Physics, and Dr. A. Ryder, School of Chemistry.
- Sept 07: Analyze-IQ software page added. See details about our machine-learning spectral analysis software..
- Dec. 07: The NBL has just been awarded funding to acquire a Total Internal reflection Fluorescence (TIRF) Microscope from Science Foundation Ireland. This will considerably enhance our ability to measure dynamic processes on the nanoscale.
- Oct 07: The NBL has been part funded under the National Biophotonics Imaging Platform (a collaborative advanced imaging consortium). As part of our contribution we will collaborate with partners in the RCSI and other institutions by providing our expertise and instrumentation in FCS, FCCS and other advanced fluorescence methods. The NUIG element is a collaboration between Prof. Peter Dockery of the Department of Anatomy, Prof. Chris Dainty, Applied Optics, Department of Experimental Physics, and Dr. A. Ryder, School of Chemistry.
- Sept 07: Analyze-IQ software page added. See details about our machine-learning spectral analysis software..
- August 06: The 30th Annual Symposium of the Microscopical Society of Ireland took place in NUI-Galway between the 30th August and the 1st of September 2006.
Details can be found on: http://www.nuigalway.ie/msi/symposium.htm - July 05: The group is pleased to announce the commencement of a very large scale collaborative research project with Dublin City University and Bristol-Myers Squibb. The project will focus on the development of new rapid analytical tools for Biopharmaceutical analysis. More information will be made available in September/October. As part of this project the group is commissioning a new laboratory in the Physical Chemistry section of the Department of Chemistry in which this project will be housed. Press releases are available from the Irish Times, Galway advertiser online and in pdf format, and PharmaManufacturing.com,
- June 05: The upright FLIM microscope has how been installed and is operational, more details of its capabilities are available here.
- May 05: The group has been awarded an SFI research frontiers grant to develop novel time-resolved fluorescence methods for analysing hydrocarbon bearing fluid inclusions (HCFI).
- April 05: Two UREKA and one STAR awards were made to the group. Under these schemes 2 undergraduate students and a secondary school teacher were based in our laboratory during the summer of 2005.
- April 2005: The inverted FLIM-FCS facility has now been installed and the first measurements are being made, more details to follow.
- Nov. 2004: Funding has been secured for Fluorescence Correlation Spectroscopy and Fluorescence Lifetime Imaging Microscopy (FLIM-FCS) facilities.
- Oct. 2004: Three postdoctoral researchers (Dr. Denisio Togashi, Dr. Patrick Fournet, and Dr. Marc Leger) have just joined the group. More details are available on the postdoc page.
- June 2004: The Fourth Stokes Summer School, Skreen, Co. Sligo, Ireland, was held over the 18-22 June weekend. The event was well attended and covered a wide range of fluorescence and luminescence topics. I will add photos from the school at a later date.
- June 2004: Two UREKA awards were made to the group. Under this scheme 2 undergraduate students were based in our laboratory for 8 weeks during the summer of 2004.
- June 2004: The group was awarded an SFI STARs for the summer of 2004. Under this scheme Yvonne Higgins a secondary school science teacher undertook a research project in the Nanoscale Biophotonics laboratory for 8 weeks on lifetime based pH sensing. Press article here.








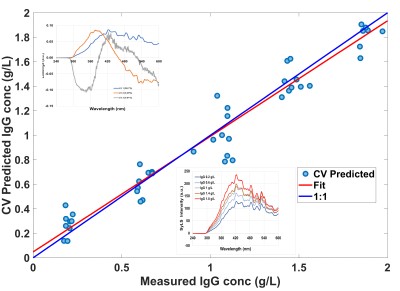 Using Rayleigh-Mie Scattered Light and Polarised Multidimensional Fluorescence Emission in Combination for Protein Quantification in a Model Clarified Bioreactor Harvest. B.O. Boateng and A.G. Ryder.
Using Rayleigh-Mie Scattered Light and Polarised Multidimensional Fluorescence Emission in Combination for Protein Quantification in a Model Clarified Bioreactor Harvest. B.O. Boateng and A.G. Ryder.  Latest addition to the lab is a
Latest addition to the lab is a  Latest addition to the lab is a Nanoparticle Tracking Analysis (NTA) system which we have for the next month or so.
Latest addition to the lab is a Nanoparticle Tracking Analysis (NTA) system which we have for the next month or so. This is the new TFF system that was installed in the lab last month.
This is the new TFF system that was installed in the lab last month.  We've now got our second
We've now got our second  Using PolA-TEEM enables us to extract several additional layers of information from complex biologics. We're using our two PolA-TEEM-DLS workstations to study a variety of interesting systems including protein-liposome interactions, high concentration protein formulations, viral inactivation processes, mAb and polymer aggregation, and anti-body drug conjugates (ADCs).
Using PolA-TEEM enables us to extract several additional layers of information from complex biologics. We're using our two PolA-TEEM-DLS workstations to study a variety of interesting systems including protein-liposome interactions, high concentration protein formulations, viral inactivation processes, mAb and polymer aggregation, and anti-body drug conjugates (ADCs).  The lab has just been upgraded with a second Dynamic Light Scattering (DLS) system.
The lab has just been upgraded with a second Dynamic Light Scattering (DLS) system. 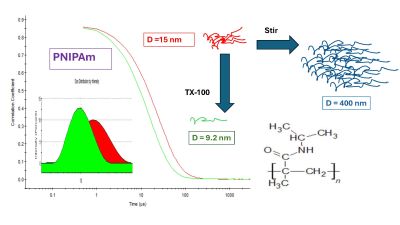 Abstract: Poly-N-isopropylacrylamide (PNIPAm), a thermorresponsive polymer, highly soluble in water below its lower critical solution temperature (LCST), is widely used in biomedical applications like drug delivery. Being able to measure PNIPAm size and aggregation state in solution quickly, inexpensively, and accurately below the LCST is critical when stoichiometric particle or molecular ratios are required. Dynamic light scattering (DLS) is probably the most widely available, and inexpensive nanoparticle sizing technique, but there are limitations with respect to sample polydispersity. Here, we first investigated factors governing the ability of DLS to accurately measure PNIPAm size in solution at 25 °C as part of a quality study of five different molecular weight, commercially sourced PNIPAm.
Abstract: Poly-N-isopropylacrylamide (PNIPAm), a thermorresponsive polymer, highly soluble in water below its lower critical solution temperature (LCST), is widely used in biomedical applications like drug delivery. Being able to measure PNIPAm size and aggregation state in solution quickly, inexpensively, and accurately below the LCST is critical when stoichiometric particle or molecular ratios are required. Dynamic light scattering (DLS) is probably the most widely available, and inexpensive nanoparticle sizing technique, but there are limitations with respect to sample polydispersity. Here, we first investigated factors governing the ability of DLS to accurately measure PNIPAm size in solution at 25 °C as part of a quality study of five different molecular weight, commercially sourced PNIPAm. Thanks to the folks at
Thanks to the folks at 
 Congratulations to
Congratulations to  Congratulations to
Congratulations to  Marina Steiner-Browne, Ana Luiza de Farie e Silva, and Camila van Zanten were all conferred with their PhD awards virtually on Tuesday afternoon. Congrats to everyone, must be a first with three Brazilian PhD students, all of who worked on various aspects of protein analysis using fluorescence spectroscopy.
Marina Steiner-Browne, Ana Luiza de Farie e Silva, and Camila van Zanten were all conferred with their PhD awards virtually on Tuesday afternoon. Congrats to everyone, must be a first with three Brazilian PhD students, all of who worked on various aspects of protein analysis using fluorescence spectroscopy.


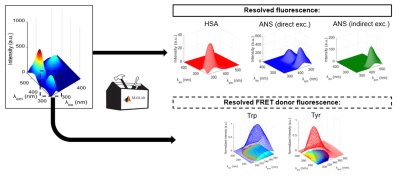
 The study of protein chemical modification using polarized Excitation Emission Matrix (pEEM).
The study of protein chemical modification using polarized Excitation Emission Matrix (pEEM).  Congratulations to Marina Steiner-Browne, a fourth year PhD student in the group who was awarded joint first prize in the annual
Congratulations to Marina Steiner-Browne, a fourth year PhD student in the group who was awarded joint first prize in the annual 

 Congratulations to Dr. Yannick Casamayou Boucau (2nd from the right) who won one of the poster prizes at the
Congratulations to Dr. Yannick Casamayou Boucau (2nd from the right) who won one of the poster prizes at the  Congratulations to Ana-Luiza da Faria e Silva (3rd from left), a third year PhD student in the group who won second prize in the student poster competition at the annual
Congratulations to Ana-Luiza da Faria e Silva (3rd from left), a third year PhD student in the group who won second prize in the student poster competition at the annual  Congratulations to Marina Steiner-Browne, a third year PhD student in the group who won second prize in the annual
Congratulations to Marina Steiner-Browne, a third year PhD student in the group who won second prize in the annual 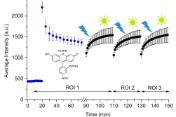


 Congratulations to Yannick Casamayou-Boucau (third from the left in picture), a third year PhD student in the group who won second prize in the annual
Congratulations to Yannick Casamayou-Boucau (third from the left in picture), a third year PhD student in the group who won second prize in the annual  Congratulations to Marina Steiner-Browne, a second year PhD student who won the best poster prize at the
Congratulations to Marina Steiner-Browne, a second year PhD student who won the best poster prize at the 







