-
Courses

Courses
Choosing a course is one of the most important decisions you'll ever make! View our courses and see what our students and lecturers have to say about the courses you are interested in at the links below.
-
University Life

University Life
Each year more than 4,000 choose University of Galway as their University of choice. Find out what life at University of Galway is all about here.
-
About University of Galway

About University of Galway
Since 1845, University of Galway has been sharing the highest quality teaching and research with Ireland and the world. Find out what makes our University so special – from our distinguished history to the latest news and campus developments.
-
Colleges & Schools

Colleges & Schools
University of Galway has earned international recognition as a research-led university with a commitment to top quality teaching across a range of key areas of expertise.
-
Research & Innovation

Research & Innovation
University of Galway’s vibrant research community take on some of the most pressing challenges of our times.
-
Business & Industry

Guiding Breakthrough Research at University of Galway
We explore and facilitate commercial opportunities for the research community at University of Galway, as well as facilitating industry partnership.
-
Alumni & Friends

Alumni & Friends
There are 128,000 University of Galway alumni worldwide. Stay connected to your alumni community! Join our social networks and update your details online.
-
Community Engagement

Community Engagement
At University of Galway, we believe that the best learning takes place when you apply what you learn in a real world context. That's why many of our courses include work placements or community projects.
Features
BEST IN CLASS: University Hospital Galway Implements New Efficient Process for Clinical Research Contracts, Overcoming Delays in Study Start-Up
Studies have shown that budget and contract negotiations are significant contributors to prolonged study start-up times worldwide. (1)(2) These delays can hinder the progress of critical research and negatively impact institutions' reputations and operational efficiency.
In early 2023, University Hospital Galway faced significant delays in contract signoffs, with turnaround times stretching from six months to two years. These delays severely impacted the hospital, leading to the loss of valuable studies and damaging its reputation within the research community. Recognizing the urgent need for improvement, the Clinical Research and Development Office (CRDO) conducted a comprehensive review of all Clinical Trials contracts submitted to their office. This review aimed to identify the root causes of the delays and develop effective solutions to streamline the process and expedite study start-ups.
The review identified several key barriers contributing to delays:
- Insufficient knowledge regarding the necessary documents for a comprehensive contract review (e.g., Protocol, Participant Information Leaflets, Data Protection Impact Assessment).
- Complexity of data protection requirements.
- Limited understanding of Clinical Indemnity Scheme (CIS) cover requirements.
- Multiple communications from various individuals concerning the same Clinical Trial Agreement (CTA).
- Absence of agreed timelines with the HSE legal team, resulting in prolonged delays.
- Lack of standardised templates.
- Overall absence of a streamlined process
To address these issues, the CRDO implemented a new process with the following solutions:
- Introduction of a new online application portal for all study contracts requiring review and sign-off, including guidance on required documents based on study type or GDPR requirements, particularly for Clinical Trials involving data transfer outside the EEA.
- Establishment of agreed processes and timelines with the HSE legal team and AON.
- Provision of comprehensive guidance documents and training sessions for researchers.
- Mandatory use of National HSE-approved Clinical Trial Agreement Templates for Investigational Medicinal Product (IMP) trials.
- Encouragement for sponsors to utilise the same template previously reviewed by the HSE legal team for all future studies.
- Collaboration with partners in other HSE hospitals to share contract reviews.
The effectiveness of the newly implemented process is clearly demonstrated by the metrics for 2023 and 2024. In 2023, the average turnaround time for all contracts (N=52) was reduced to 32 working days. This efficiency further improved in 2024, with the average turnaround time for all contracts (N=59) currently standing at 29 days. Specifically, for clinical trials, an impressive 97% of contracts have been executed in less than 14 weeks in 2024.
These significant improvements underscore the success of the streamlined process in several key areas:
- Reducing Delays: The new process has effectively minimized the delays that previously plagued contract signoffs, ensuring a more timely progression of clinical research projects.
- Enhancing Efficiency: By standardizing procedures and providing clear guidance, the hospital has enhanced the overall efficiency of contract reviews and approvals.
- Expediting Study Start-Ups: The expedited turnaround times have facilitated quicker study start-ups, allowing research to commence without unnecessary delays.
Overall, these advancements highlight University Hospital Galway's commitment to excellence in clinical research and its proactive approach to overcoming operational challenges. The hospital's new process sets a benchmark for efficiency and effectiveness in clinical research contract management.
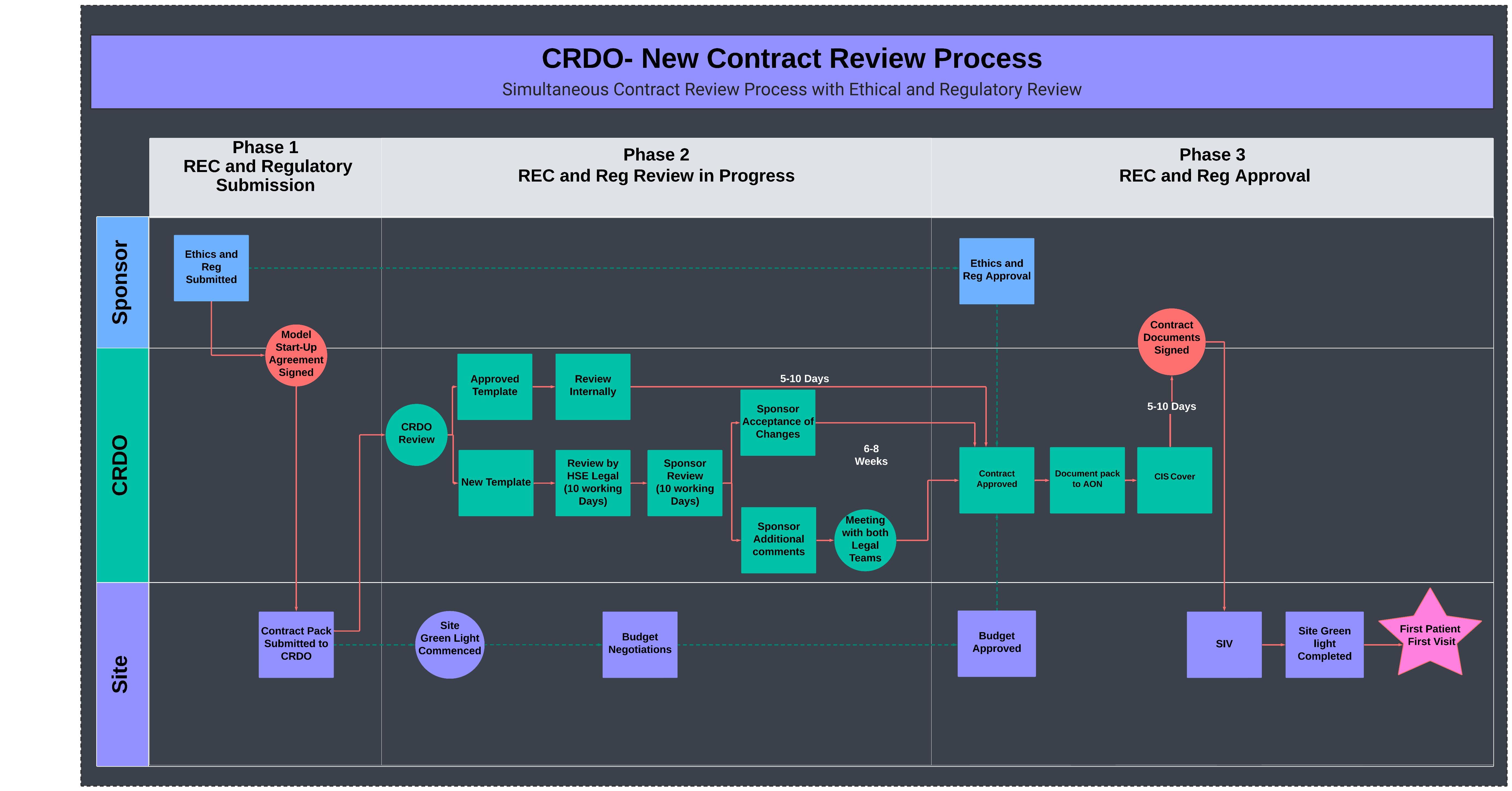
In 2024, we have further refined our process with the introduction of the new Model Study Start-Up Agreement. This innovative agreement allows us to initiate our contract review concurrently with the ethics and regulatory authorities' review of the clinical trial.
The Model Study Start-Up Agreement provides several key benefits:
- Simultaneous Reviews: By aligning the contract review process with the ethics and regulatory reviews, we can significantly reduce the overall time required to commence a clinical trial.
- Cost Assurance: The agreement ensures that any costs incurred by the site, such as those for contract review and budget negotiations, are reimbursed even if the clinical trial does not receive approval. This provision offers financial security to the site and encourages timely engagement in the review process.
- Expedited Study Openings: Although the review timeline itself remains unchanged, the concurrent review process expedites the overall study start-up at the site, allowing research activities to begin more swiftly.
The process flow map for the Model Study Start-Up Agreement figure 1, outlines the streamlined steps and interactions involved in this enhanced process.
These enhancements reflect our ongoing commitment to improving efficiency and effectiveness in clinical research contract management, ensuring that University Hospital Galway remains at the forefront of clinical research excellence.
Figure 1
References
[1] Study Start-Up Timelines: Identifying Challenges & Opportunities ... - CTTI
[2] CTTI Report Finds Contracting and Budgeting are Significant Pain Points ...








.jpeg)







